Pfizer Plans To Submit Vaccine Data For Youngest Children By Early June
May 3, 2022 10:25AM PDT
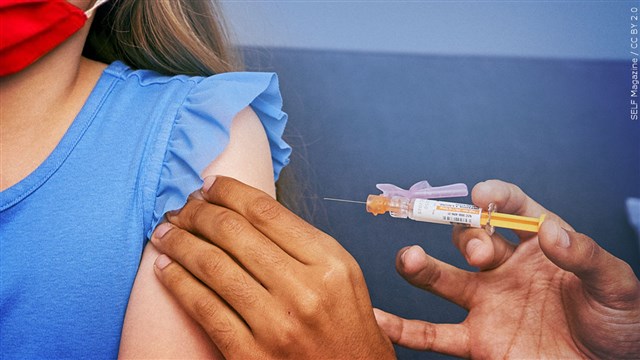
ATLANTA, Ga. (AP) – = Pfizer now hopes to tell U.S. regulators how well its COVID-19 vaccine works in the littlest kids by early June.
Currently only children ages 5 or older can be vaccinated in the U.S., using Pfizer’s vaccine.
Rival Moderna hopes to be the first to offer vaccinations to the youngest children, and began filing its own data with the Food and Drug Administration last week.
The FDA has set tentative meetings in June to review data from one or both companies.
More about:
You Might Also Like



