FDA Approves Over-The-Counter Narcan
March 29, 2023 9:51AM PDT
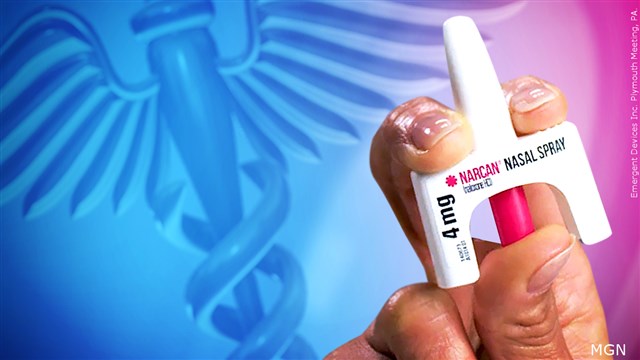
Credit: MGN
(ASSOCIATED PRESS) – The U.S. Food and Drug Administration has approved selling overdose antidote naloxone over-the-counter, marking the first time an opioid treatment drug will be available without a prescription.
Wednesday’s approval is for Narcan, a name-brand version of naloxone sold by Emergent BioSolutions.
How much this will impact a nationwide overdose crisis is not clear, even though better access to naloxone is a priority.
The decision means Narcan can be available at convenience and grocery stores, but its price isn’t clear.
For many people who use drugs, naloxone is already available from community groups — and that’s not expected to change.
More about:



